
- #4D MOLECULAR THERAPEUTICS SALARY FULL#
- #4D MOLECULAR THERAPEUTICS SALARY TRIAL#
- #4D MOLECULAR THERAPEUTICS SALARY PLUS#
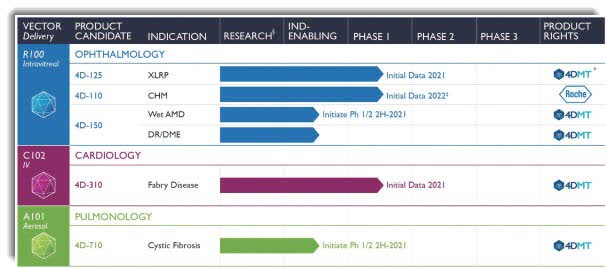
We therefore expect to potentially develop 4D-710 in this broader patient population, as a single agent and/or in combination with these CFTR modulator small molecule medicines.Ĭystic fibrosis is a major inherited disease caused by mutations in the CFTR gene. In patients with CFTR mutations whose disease is amenable to modulator medicines, the improvement in lung function is variable. 4D-710 is being initially developed in the approximately 10-15% of patients whose disease is not amenable to existing medicines targeting the CFTR protein. 4D-710 has the potential to treat a broad range of patients with cystic fibrosis, independent of the specific CFTR mutation, and is designed for aerosol delivery to achieve CFTR expression within lung airway epithelial cells.
#4D MOLECULAR THERAPEUTICS SALARY PLUS#
Secondary endpoints include assessments of clinical activity including lung function, plus exploratory endpoints on the feasibility of detecting transgene transfer and microCFTR expression as measured in bronchoscopic biopsies and brushings.ĤD-710 is comprised of our targeted and evolved vector, A101, and a codon-optimized microCFTR transgene. The primary endpoint of the study is safety and tolerability. In the dose-escalation phase, two dose levels of 4D-710 will be examined in a 3+3 design.

#4D MOLECULAR THERAPEUTICS SALARY TRIAL#
The Phase 1/2 clinical trial is a multicenter, open-label, dose-escalation and dose-expansion trial of 4D-710 in patients (n=~18) with cystic fibrosis who are ineligible for CFTR modulator therapy or who have discontinued therapy due to adverse effects. It could benefit both people with cystic fibrosis who aren’t able to take CFTR modulators as well as those who have a substantial residual deficit in lung function in spite of modulator therapy.” “This therapy has the potential to treat a broad range of people with cystic fibrosis independent of their specific CFTR mutations. Taylor-Cousar, M.D., M.S.C.S, Professor of Medicine and Pediatrics at National Jewish Health and lead principal investigator for the Phase 1/2 clinical trial. “4D-710 is designed for aerosol delivery to achieve CFTR expression within lung airway epithelial cells,” said Jennifer L.
#4D MOLECULAR THERAPEUTICS SALARY FULL#
We are seeking to unlock the full potential of genetic medicines through our platform and to fulfill the promise of transformative biotherapeutics to benefit patients.” To date, our platform has produced five clinical-stage product candidates that incorporate three different proprietary and novel capsids. “4D-710 utilizes the aerosol-delivered A101 vector developed at 4DMT through our proprietary Therapeutic Vector Evolution platform. “The dosing of the first patient in the 4D-710 Phase 1/2 clinical trial in cystic fibrosis marks an important milestone for our company and for the patients we aim to benefit,” said Robert Fishman, M.D., Chief Medical Officer and Pulmonology Therapeutic Area Head of 4DMT. (4DMT) (Nasdaq: FDMT), a clinical-stage biotherapeutics company harnessing the power of directed evolution for targeted genetic medicines, announced that the first patient has been dosed in its Phase 1/2 clinical trial of 4D-710 in patients with cystic fibrosis. The visa approval numbers from consular processing are provided by Depart of State (DoS) and can be reviewed on website.EMERYVILLE, Calif., Ap(GLOBE NEWSWIRE) - 4D Molecular Therapeutics, Inc. If you are outside country, then employer will ask the H1B worker to attend visa interview at the nearest consulate. USCIS data is only for folks who are already in the USA and requesting for change of status (COS). USCIS review the H1B applications (new, renewals, and transfer of employer) and can result in approval or denial. Once DOL approves LCA, the petition is submitted to USCIS for review. On the other hand USCIS data indicates, how much success does an employer have in getting the LCAs to hiring those foreign workers. Read more about LCA process in blog article. In other words, filing an LCA and getting an approval is a mandatory step for H1B visa. One LCA application can be used to fulfil many open positions by an employer. These applications are either new employment, continuation of the employment or change of employers.
LCA data represents the number of applications filed with Department Of Labor (DOL) request to hire a foreign worker. Below is the charts and tables of Labor Condition Application (LCA) approvals and denials along with USCIS approvals and denials.


 0 kommentar(er)
0 kommentar(er)
